Repensando la pioglitazona como agente cardioprotector: una nueva perspectiva
¿Por qué la pioglitazona deber ser considerada un fármaco cardioprotector?
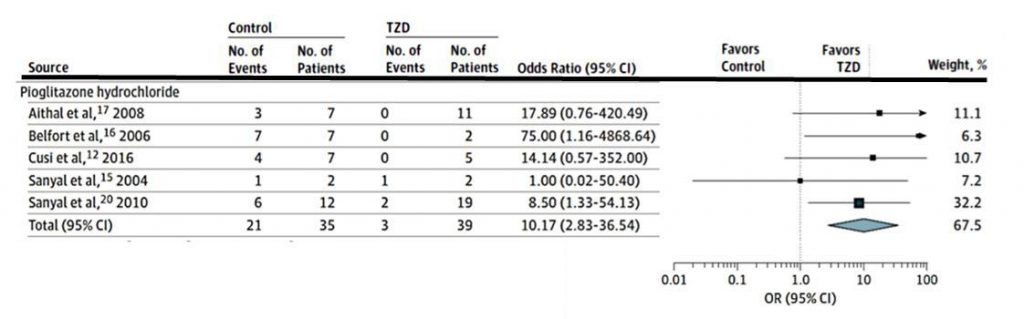
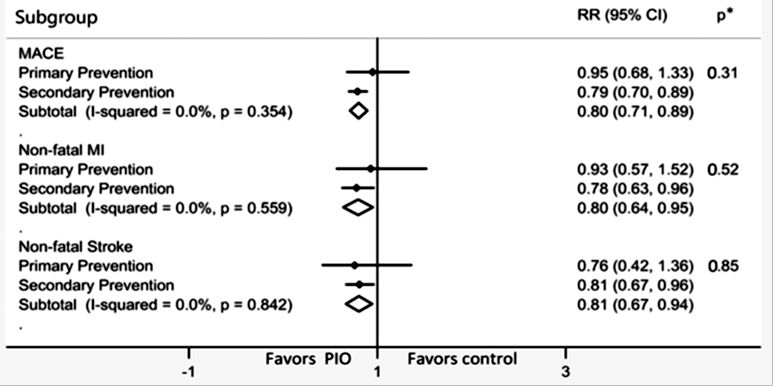
Numerosos estudios han demostrado que la Pioglitazona disminuye el riesgo MACE, de infarto agudo de miocardio y de ACV. Ya en el año 2005 el estudio PROACTIVE demostró una reducción del 16% del compuesto Muerte + IAM no fatal + ACV no fatal (HR 0.84, 95% CI 0.72–0.98). En el año 2017, un metaanálisis que inluyó 12.026 pacientes demostró una reducción de riesgo de MACE del 23% (HR 0.77, IC 0.64–0.93) para pacientes con prediabetes y del 17% (0.83, 0.72–0.97) para pacientes con diabetes. Un nuevo metaanálisis de 26 estudios controlados (N= 19.645) en 2020 confirmó una reducción del riesgo de MACE del 20% (HR 0.80, IC 0.71-0.89) (ver figura 1)
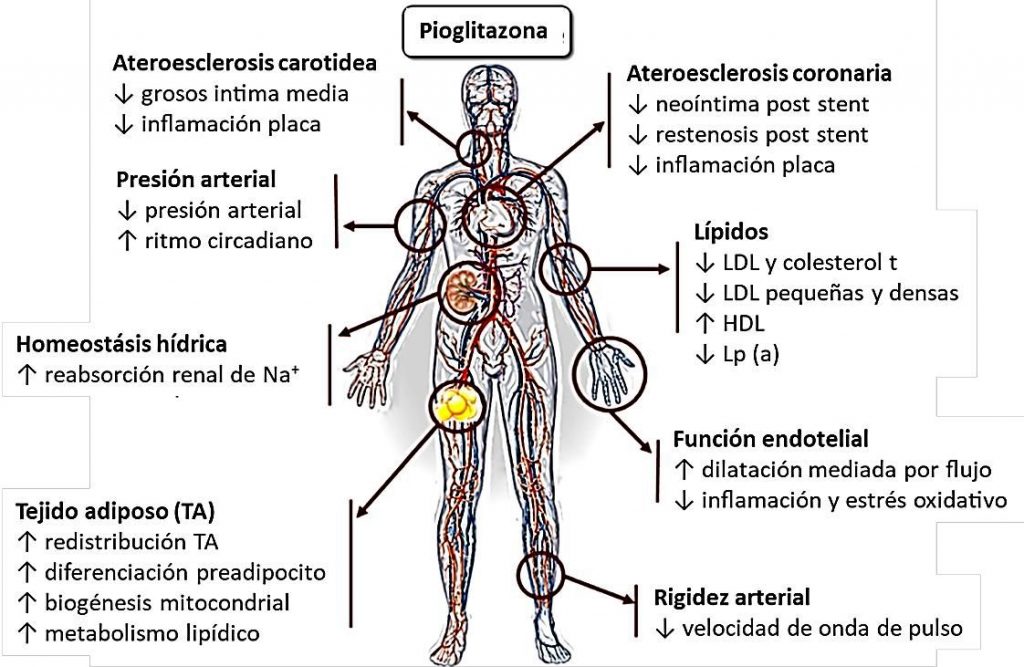
¿Qué efectos fueron estudiados relacionados con la protección vascular?
La protección cardiovascular observada con pioglitazona se puede deber a diversos mecanismos resumidos en la figura 2.
¿En qué pacientes debemos contraindicar su uso?
Las evidencias actuales contraindican su uso en pacientes con antecedentes de Insuficiencia cardiaca clase funcional III-IV ya que la pioglitazona podría aumentar el riesgo de internaciones.
MACE: eventos cardiovasculares mayores (mortalidad cardiovascular, infarto no fatal y ACV no fatal)
Trabajo completo publicado en Nesti et al. Cardiovasc Diabetol (2021) 20:109. https://doi.org/10.1186/s12933-021-01294-7